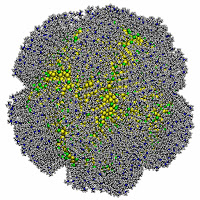
This post has been a long tine coming... I wrote it back in May 2015, and somehow in the middle of things, I forgot to hit "publish." While we have done quite a bit of work with this model since then, maybe you'll still enjoy our crazy analogy to playing dice with particles at the mesoscale...
Some time ago, I published what might seem as yet another paper describing the properties of our model for (coarse-grained) large-scale macromolecules. A critical part of the model is that we roll dice every time these particles collide so as to decide whether they bounce or go through each other. They can overlap, because at intermediate length scales, they don't behave like rocks even if they occupy space. Despite our simple (and dicey) model, in our earlier papers, we showed that our particles give rise to the same structure as the corresponding particles that would interact through typical (so-called soft) interactions. But Einstein's famous quote about God not playing dice with the universe (albeit in a different context) serves as a warning that our particles might not move in analogous ways to those driven by Newton's deterministic laws. In our most recent paper, we confirmed that our particles (if they live in one dimension) do recover deterministic dynamics at sufficiently long (that is, coarse-grained) length scales. That's a baby step towards using our model in human-scale (three) dimensions. So there are more papers to come!
The work was performed (and the paper was written) with my recent Ph.D. graduate, Dr. Galen Craven, and a Research Scientist, Dr. Alex Popov. It's basic research and I'm happy to say that It was supported by the National Science Foundation. The title of the article is "Stochastic dynamics of penetrable rods in one dimension: Entangled dynamics and transport properties," and it was recently published at J. Chem. Phys. 142, 154906 (2015).
Showing posts with label publications. Show all posts
Showing posts with label publications. Show all posts
Sunday, March 24, 2019
Sunday, March 17, 2019
Engineered gold nanoparticles can be like ice cream scoops covered in chocolate sprinkles
There are many ways to interrogate molecular phenomenon. You might think that this is restricted to physical measurements such as direct observation with a microscope, a laser, or more seemingly arcane observation with nuclear magnetic resonance (NMR). But I’m happy to report that observation of computer simulations is yet another, as long as our models are sufficiently accurate that they mimic reality perfectly. In today’s age when it’s hard to see the difference between CGI and real humans, this may not sound surprising. Nevertheless, the question is what can we learn from observation of real and simulated systems in tandem?
I’m happy to report that my student Gene Chong and Cathy Murphy’s student Meng Wu did precisely this parallel study. Gene made simulations of a simpler system, involving nanoparticles covered by lipids called MUTABs. Meng made NMR measurements of nanoparticles covered by a similar but somewhat longer lipids called MTABs. (Note that if you are worried about the term nuclear in NMR, as in nuclear energy, don’t be. We are just looking at the positions of the nuclei, not spitting them apart. It was the concern over this misunderstanding that led to the use of such a device to look at your body in detail to be called MRI instead of NMR!) The happy result was that the two observations agreed. But only together did Meng’s and Gene’s observations show clearly that the lipids didn’t always cover the nanoparticle smoothly like melted chocolate on ice cream, but rather assembled like sprinkles all pointing out in the same direction packed together in different islands on the surface. This structure means that lipid-decorated nanoparticles will have shape and response to other systems that you might not otherwise anticipate. And this opens the question to our next set of investigations as we chart a course to understand the interactions between nanoparticles and biological components such as membranes.
If you want more detail, check out our article in JACS, just recently published! That is, JACS 141, 4316 (2019), and I'm happy to report that it was funded by the NSF CCI program for our Center for Sustainable Nanotechnology.
I’m happy to report that my student Gene Chong and Cathy Murphy’s student Meng Wu did precisely this parallel study. Gene made simulations of a simpler system, involving nanoparticles covered by lipids called MUTABs. Meng made NMR measurements of nanoparticles covered by a similar but somewhat longer lipids called MTABs. (Note that if you are worried about the term nuclear in NMR, as in nuclear energy, don’t be. We are just looking at the positions of the nuclei, not spitting them apart. It was the concern over this misunderstanding that led to the use of such a device to look at your body in detail to be called MRI instead of NMR!) The happy result was that the two observations agreed. But only together did Meng’s and Gene’s observations show clearly that the lipids didn’t always cover the nanoparticle smoothly like melted chocolate on ice cream, but rather assembled like sprinkles all pointing out in the same direction packed together in different islands on the surface. This structure means that lipid-decorated nanoparticles will have shape and response to other systems that you might not otherwise anticipate. And this opens the question to our next set of investigations as we chart a course to understand the interactions between nanoparticles and biological components such as membranes.
If you want more detail, check out our article in JACS, just recently published! That is, JACS 141, 4316 (2019), and I'm happy to report that it was funded by the NSF CCI program for our Center for Sustainable Nanotechnology.
Tuesday, March 5, 2019
Deleted edits from my Comment on diversity and inclusion

When writing a Comment in any magazine, you invariably have a word limit. It's also important to stay on message so that readers won't get lost in the weeds. Thankfully, C&EN has great editors, and it was a pleasure to work with them to write my recent Comment (details at bottom) to help me stay on message. Indeed, while I appreciate the power of blogging and writing unfettered, there is great value in a strong editor. I am thankful that we still have them in the publishing world. In case, though, you want to see some of the weeds that got cut out, here's your sneak peak:
"An evocative triple or triad for managing diversity and advancing inclusive excellence is Support. Compliance, and Adjudication."
"Compliance is necessary in today’s world because we have seen that without it, systems tend to move out of whack, but it tends to be seen to be about protecting an administration from legal attacks."
"Adjudication provides a mechanism to resolve conflicts between aggrieved parties, but who decides which party is in the right or wrong, and how can we be sure that they are fully supporting the individuals fairly."
"Does a member trust us an authoritative source of information? Does a member trust us to prepare them throughout their professional career progression? Does a member trust us even when we tell them that they are wrong? If we are to foster a healthy diversity culture within our Society, we need to be able to do all of this while still being a single organization."
"This [a workshop held at Barnard] included 1-point gains on a 5-point scale on four of the six objectives: (1) the difference and importance of transactional solutions vs. policy solutions as it pertains to managing diversity equity and inclusion, (2) factors for administering recruitment, mentoring, tenure and promotion processes that advance inclusive excellence, (3) evidence-based strategies for addressing known barriers within a department so as to reduce existing diversity inequities, and (4) supporting and communicating inclusive excellence. Our targeted learning outcomes clearly resonate with the three legs of our managing diversity table and our approach to managing them. "
"The perhaps surprising outcome is that the practices necessary to manage diversity are simply the applications of good management to achieve a targeted outcome, which in this case is inclusive excellence."
"The courses offered by the ACS through the Leadership Advisory Board (LAB) where essential to me as I have developed as a leader, and I’m happy to recommend then to you so as to learn the underlying principles of management."
I also hope that you have a chance to read my recent Comment in C&EN on "Bringing diversity and inclusion to the ACS table." (Volume 97, Issue 9, March 2, 2019) If not, please check it out. Access is free if you are an ACS member. Otherwise, you can use one of your 5 free monthly views... And if you are a chemist, please consider joining.
Wednesday, September 30, 2015
Reimagining the geometry of transition states (in PRL!)
Specifically, we discovered a new way for obtaining the structure of the transition state between reactants and products when the reaction is in a complex solvent. All of the previous methods had obtained this surface by optimization (using variational transition state theory) or through successive approximations (using perturbation theory).The key is a mathematical tool, called the Lagrangian descriptor, that had been developed earlier by Wiggins and his colleagues in the area of fluid mechanics.We were able to use the Lagrangian descriptor to obtain the transition state geometry directly without either optimizing the rate or employing perturbation theory. And this means that we now have a new tool for obtaining reaction rates in nonequilibrium systems.
As with most articles in PRL, it was a tortuous path through the reviewing process. We were pleased that nearly all of the reviewers (and we had 6 in the end!) saw the work as novel and potentially game-changing. The full reference of the article is: G. T. Craven and R. Hernandez, "Lagrangian descriptors of thermalized transition states on time-varying energy surfaces," Phys. Rev. Lett. 115, 148301 (2015). (doi:10.1103/PhysRevLett.115.148301) I'm happy to acknowledge the support from the Air Force Office of Scientific Research (AFOSR).
Tuesday, June 16, 2015
Sustainable Nano on Open Access Sustainably
(This article is a cross-post between EveryWhereChemistry and Sustainable-Nano!)
Sustainability’s future is now. Our recent article was just published in an all-electronic journal, ACS Central Science, which is among the first of the American Chemical Society (ACS) journals offered without a print option. It therefore embodies sustainability as it requires no paper resources, thereby limiting the journal’s carbon footprint to only what is required for maintaining the information electronically in perpetuity. It is also completely Open Access, which means our article is available for all to read. Does this equal accessibility (called “flat” because there is no hierarchy in levels of access) amount to yet another layer of sustainability? More on that question in a moment. Meanwhile as the article itself is about sustainability, it embodies the repetitive word play in the title of this post.
But there is another double meaning in the publishing of this work: The flatness underlying the vision of Open Access is also at play in how the work was done. ELEVEN different research groups were involved in formulating the ideas and writing the paper. This lot provided tremendous breadth of expertise, but the flatness in the organizational effort allowed us to merge it all together. Of course, it wouldn’t have happened without significant leadership, and Cathy Murphy, the paper’s first author, orchestrated us all magnificently. While flatness in organizational behavior isn’t typically considered part of sustainability, in this case it provided for the efficient utilization of resources (that is, ideas) across a broader cohort.
So what is our article about? Fifteen years into the 21st century, it is becoming increasingly clear that we need to develop new materials to solve the grand challenges that confront us in the areas of health, energy, and the environment. Nanoparticles are playing a significant role in new material development because they can provide human-scale effects with relatively small amounts of materials. The danger is that because of their special properties, the use of nanoparticles may have unintended consequences. Thus, many in the scientific community, including those of us involved in writing this article, are concerned with identifying rules for the design and fabrication of nanoparticles that will limit such negative effects, and hence make the particles sustainable by design. In our article, we propose that the solution of this grand challenge hinges on four critical needs:
1. Chemically Driven Understanding of the Molecular Nature of Engineered Nanoparticles in Complex, Realistic Environments
2. Real-Time Measurements of Nanomaterial Interaction with Living Cells and Organisms That Provide Chemical Information at Nanometer Length Scales To Yield Invaluable Mechanistic Insight and Improve Predictive Understanding of the Nano−Bio Interface.
3. Delineation of Molecular Modes of Action for Nanomaterial Effects on Living Systems as Functions of Nanomaterial Properties
4. Computation and Simulation of the Nano−Bio Interface.
In more accessible terms, this translates to: (1) It’s not enough to know how the nanoparticles behave in a test tube under clean conditions as we need to know how they might behave at the molecular scale in different solutions. (2) We also need to better understand and measure the effects of nanoparticles at contact points between inorganic materials and biological matter. (3) Not only do we need to observe how nanoparticles behave in relation to living systems, but to understand what drives that behavior at a molecular level. (4) In order to accelerate design and discovery as well as to avoid the use of materials whenever possible, we also need to design validated computational models for all of these processes.
Take a look at the article for the details as we collectively offer a blueprint for what research problems need to be solved in the short term (a decade or so), and how our team of nanoscientists, with broad experience in making, measuring, and simulating nanoparticles in complex environments, can make a difference.
The title of the article is "Biological Responses to Engineered Nanomaterials: Needs for the Next Decade.” The work was funded by the NSF as part of the Phase I Center for Sustainable Nanotechnology (CSN, CHE-124051). It was just released at ACS Central Science, XXXX (2015) as an ASAP Article. The author list is C. Murphy, A. Vartanian, F. Geiger, R. Hamers, J. Pedersen, Q. Cui, C. Haynes, E. Carlson, R. Hernandez, R. Klaper, G. Orr, and Z. Rosenzweig,
It’s available as Open Access right now at http://dx.doi.org/10.1021/acscentsci.5b00182
Sustainability’s future is now. Our recent article was just published in an all-electronic journal, ACS Central Science, which is among the first of the American Chemical Society (ACS) journals offered without a print option. It therefore embodies sustainability as it requires no paper resources, thereby limiting the journal’s carbon footprint to only what is required for maintaining the information electronically in perpetuity. It is also completely Open Access, which means our article is available for all to read. Does this equal accessibility (called “flat” because there is no hierarchy in levels of access) amount to yet another layer of sustainability? More on that question in a moment. Meanwhile as the article itself is about sustainability, it embodies the repetitive word play in the title of this post.
But there is another double meaning in the publishing of this work: The flatness underlying the vision of Open Access is also at play in how the work was done. ELEVEN different research groups were involved in formulating the ideas and writing the paper. This lot provided tremendous breadth of expertise, but the flatness in the organizational effort allowed us to merge it all together. Of course, it wouldn’t have happened without significant leadership, and Cathy Murphy, the paper’s first author, orchestrated us all magnificently. While flatness in organizational behavior isn’t typically considered part of sustainability, in this case it provided for the efficient utilization of resources (that is, ideas) across a broader cohort.
So what is our article about? Fifteen years into the 21st century, it is becoming increasingly clear that we need to develop new materials to solve the grand challenges that confront us in the areas of health, energy, and the environment. Nanoparticles are playing a significant role in new material development because they can provide human-scale effects with relatively small amounts of materials. The danger is that because of their special properties, the use of nanoparticles may have unintended consequences. Thus, many in the scientific community, including those of us involved in writing this article, are concerned with identifying rules for the design and fabrication of nanoparticles that will limit such negative effects, and hence make the particles sustainable by design. In our article, we propose that the solution of this grand challenge hinges on four critical needs:
1. Chemically Driven Understanding of the Molecular Nature of Engineered Nanoparticles in Complex, Realistic Environments
2. Real-Time Measurements of Nanomaterial Interaction with Living Cells and Organisms That Provide Chemical Information at Nanometer Length Scales To Yield Invaluable Mechanistic Insight and Improve Predictive Understanding of the Nano−Bio Interface.
3. Delineation of Molecular Modes of Action for Nanomaterial Effects on Living Systems as Functions of Nanomaterial Properties
4. Computation and Simulation of the Nano−Bio Interface.
In more accessible terms, this translates to: (1) It’s not enough to know how the nanoparticles behave in a test tube under clean conditions as we need to know how they might behave at the molecular scale in different solutions. (2) We also need to better understand and measure the effects of nanoparticles at contact points between inorganic materials and biological matter. (3) Not only do we need to observe how nanoparticles behave in relation to living systems, but to understand what drives that behavior at a molecular level. (4) In order to accelerate design and discovery as well as to avoid the use of materials whenever possible, we also need to design validated computational models for all of these processes.
Take a look at the article for the details as we collectively offer a blueprint for what research problems need to be solved in the short term (a decade or so), and how our team of nanoscientists, with broad experience in making, measuring, and simulating nanoparticles in complex environments, can make a difference.
The title of the article is "Biological Responses to Engineered Nanomaterials: Needs for the Next Decade.” The work was funded by the NSF as part of the Phase I Center for Sustainable Nanotechnology (CSN, CHE-124051). It was just released at ACS Central Science, XXXX (2015) as an ASAP Article. The author list is C. Murphy, A. Vartanian, F. Geiger, R. Hamers, J. Pedersen, Q. Cui, C. Haynes, E. Carlson, R. Hernandez, R. Klaper, G. Orr, and Z. Rosenzweig,
It’s available as Open Access right now at http://dx.doi.org/10.1021/acscentsci.5b00182
Monday, May 25, 2015
Stretching proteins and myself into open access (OA)
I'm not sure where to side on the Open Access (OA) publishing business. On the one hand, paying for an article to be published is a regression to the days of page charges albeit without the double-bind that readers are also required to pay. On the other hand, it does flatten access to the article, and often panders to enlightened self-interest by way of increased exposure and citations. Indeed, a strong argument in favor of OA for articles, data and code was just published in the Journal of Chemical Physics by my friend, Dan Gezelter. (Fortunately his Viewpoint is OA and readily available.) Regardless, publishers need to cover their costs, and here lies the challenge to the scientific community. The various agencies supporting science do not appear to be increasing funding to subsidize the fees even while they are making policy decisions to require OA. Libraries love OA because it might potentially lower their skyrocketing journal costs, though no substantial lowering appears to have yet occurred. Long story short, my group is now doing the experiment: We recently submitted and just published our work in PLoS (Public Library of Science.) Props to them for being consistent as they also required us to deposit our data in a public site. I was also impressed by the reviewing process which did not appear to be lowered in any way by the presumed conflict-of-interest that a publisher might have to accept papers (and associated cash) from all submissions. The experiment continues as I'll watch to see how our OA article fares compared to our earlier articles on ASMD in more traditional journals.
Meanwhile, we are excited about the work itself. My students, led by outstanding graduate student, Hailey Bureau, validated our staged approach (called adaptive steered molecular dynamics, ASMD) to characterize the energies for pulling a protein apart. The extra wrinkle lies in the fact that the protein is sitting in a pool of water. That increases the size of the calculation significantly as you have to include the thousands (or more) of extra atoms in the pool. The first piece of good news—that we had also seen earlier—is that ASMD can be run for this system using a reasonable amount of computer time. Even better, we found that we could use a simple (mean-field) model for the water molecules to obtain nearly the same energies and pathways. This was a happy surprise because, for the most part, the atoms (particularly the hydrogens) on the protein appear to orient towards the effective solvent as if the water molecules were actually there.
Fortunately, because of OA, you can easily read the details online. The full reference to the article is: H. R. Bureau, D. Merz Jr., E. Hershkovits, S. Quirk and Rigoberto Hernandez, "Constrained unfolding of a helical peptide: Implicit versus Explicit Solvents," PLoS ONE 10, e0127034 (2015). (doi:10.1371/journal.pone.0127034) I'm also happy to say that It was supported by the National Science Foundation.
Meanwhile, we are excited about the work itself. My students, led by outstanding graduate student, Hailey Bureau, validated our staged approach (called adaptive steered molecular dynamics, ASMD) to characterize the energies for pulling a protein apart. The extra wrinkle lies in the fact that the protein is sitting in a pool of water. That increases the size of the calculation significantly as you have to include the thousands (or more) of extra atoms in the pool. The first piece of good news—that we had also seen earlier—is that ASMD can be run for this system using a reasonable amount of computer time. Even better, we found that we could use a simple (mean-field) model for the water molecules to obtain nearly the same energies and pathways. This was a happy surprise because, for the most part, the atoms (particularly the hydrogens) on the protein appear to orient towards the effective solvent as if the water molecules were actually there.
Fortunately, because of OA, you can easily read the details online. The full reference to the article is: H. R. Bureau, D. Merz Jr., E. Hershkovits, S. Quirk and Rigoberto Hernandez, "Constrained unfolding of a helical peptide: Implicit versus Explicit Solvents," PLoS ONE 10, e0127034 (2015). (doi:10.1371/journal.pone.0127034) I'm also happy to say that It was supported by the National Science Foundation.
Saturday, April 4, 2015
Balls of ice cream
When you go to your local ice cream shop, you likely ponder the question of how many scoops you wish to order. I, on the other hand, prefer to order balls of ice cream. The scoop corresponds to the void that is to be filled, but a ball corresponds to the filling. It seems to me that I'd rather order balls of ice cream rather than empty space. My wife suggests that this is just a matter of my literal mistranslation of "bolas de helado" rather than any deeper significance over the ontology of balls verses scoops. As an optimist, I certainly prefer for them to be more than half-filled, and hence naturally prefer balls of ice cream to voids, that is scoops.
This argument extends to the nanoscale. Are the properties of a particle driven by itself or by the space which it occupies? If the latter, then its properties are independent of the nanoparticle aside from its shape. As a chemist, this is a disappointing possibility because I would like to tailor the properties of the particles, such as how they assemble, by changing the atoms or molecules at the surface (and the interior). The good news is that such control can be exercised. That is why we have been studying so-called Janus particles and other patchy particles. In our rendering, they look like ice cream balls made of blueberry and strawberry ice cream halves or layers. The blueberry faces like to face the strawberry faces (because they correspond to charges and opposites attract), and this gives rise to their interesting patterns and response to changes from the outside.
Check out our some of our recent papers on Janus and striped particles and stay tuned for the next ones!
M. C. Hagy and R. Hernandez, "Dynamical simulation of electrostatic striped colloidal particles," J. Chem. Phys. 140, 034701 (2014). (doi:10.1063/1.4859855)
M. C. Hagy and R. Hernandez, "Dynamical simulation of dipolar Janus colloids: Dynamical properties," J. Chem. Phys. 138, 184903 (2013). (doi:10.1063/1.4803864)
M. C. Hagy and R. Hernandez, "Dynamical Simulation of Dipolar Janus Colloids: Equilibrium Structure and Thermodynamics," J. Chem. Phys. 137, 044505 (2012). (doi:10.1063/1.4737432)
This argument extends to the nanoscale. Are the properties of a particle driven by itself or by the space which it occupies? If the latter, then its properties are independent of the nanoparticle aside from its shape. As a chemist, this is a disappointing possibility because I would like to tailor the properties of the particles, such as how they assemble, by changing the atoms or molecules at the surface (and the interior). The good news is that such control can be exercised. That is why we have been studying so-called Janus particles and other patchy particles. In our rendering, they look like ice cream balls made of blueberry and strawberry ice cream halves or layers. The blueberry faces like to face the strawberry faces (because they correspond to charges and opposites attract), and this gives rise to their interesting patterns and response to changes from the outside.
Check out our some of our recent papers on Janus and striped particles and stay tuned for the next ones!
M. C. Hagy and R. Hernandez, "Dynamical simulation of electrostatic striped colloidal particles," J. Chem. Phys. 140, 034701 (2014). (doi:10.1063/1.4859855)
M. C. Hagy and R. Hernandez, "Dynamical simulation of dipolar Janus colloids: Dynamical properties," J. Chem. Phys. 138, 184903 (2013). (doi:10.1063/1.4803864)
M. C. Hagy and R. Hernandez, "Dynamical Simulation of Dipolar Janus Colloids: Equilibrium Structure and Thermodynamics," J. Chem. Phys. 137, 044505 (2012). (doi:10.1063/1.4737432)
Tuesday, March 31, 2015
Controlling chemical reactions by kicking their environs
Chemists dream of controlling molecular reactions with ever finer precision. At the shortest chemical length scales, the stumbling block is that atoms don’t follow directions. Instead, we “control” chemical reactions by way of putting the right molecules together in a sequence of steps that ultimately produce the desired product. As this ultimate time scale must be sufficiently short that we will live to see the product, the rate of a chemical reaction is also important. If the atoms don’t quite move in the right directions quickly enough, then we are tempted to direct them in the right way through some external force, such as from a laser or electric field. But even this extra control might not be enough to overcome the fact that the atoms forget the external control because they are distracted by the many molecules around them.
In order to control chemical reactions at the atom scale, we are therefore working to determine the extent to which chemical reaction rates can be affected by driven periodic force. Building on our recent work using non-recrossing dividing surfaces within transition state theory, we succeeded in obtaining the rates of an albeit relative simple model reaction driven by a force that is periodic (but not single frequency!) in the presence of thermal noise. It is critical that the external force is changing the entire environment of the molecule through a classical (long-wavelength) mode and not a specific vibration of the molecule through a quantum mechanical interaction. The latter had earlier been seen to provide only subtle effects at best, but the former can be enough to dramatically affect the rate and pathway of the reaction as we saw in our recent work. Thus while our most recent work is limited by the simplicity of the chosen model, it holds promise for determining the degree of control of the rates in more complicated chemical reactions.
This work was performed by my recently graduated student, Dr. Galen Craven, in collaboration with Thomas Bartsch from Loughborough University. The tile of the article is "Chemical reactions induced by oscillating external fields in weak thermal environments."The work was funded by the NSF, and the international partnership (Trans-MI) was funded by the EU People Programme (Marie Curie Actions). It was just released at J. Chem. Phys. 142, 074108 (2015).
In order to control chemical reactions at the atom scale, we are therefore working to determine the extent to which chemical reaction rates can be affected by driven periodic force. Building on our recent work using non-recrossing dividing surfaces within transition state theory, we succeeded in obtaining the rates of an albeit relative simple model reaction driven by a force that is periodic (but not single frequency!) in the presence of thermal noise. It is critical that the external force is changing the entire environment of the molecule through a classical (long-wavelength) mode and not a specific vibration of the molecule through a quantum mechanical interaction. The latter had earlier been seen to provide only subtle effects at best, but the former can be enough to dramatically affect the rate and pathway of the reaction as we saw in our recent work. Thus while our most recent work is limited by the simplicity of the chosen model, it holds promise for determining the degree of control of the rates in more complicated chemical reactions.
This work was performed by my recently graduated student, Dr. Galen Craven, in collaboration with Thomas Bartsch from Loughborough University. The tile of the article is "Chemical reactions induced by oscillating external fields in weak thermal environments."The work was funded by the NSF, and the international partnership (Trans-MI) was funded by the EU People Programme (Marie Curie Actions). It was just released at J. Chem. Phys. 142, 074108 (2015).
Friday, November 21, 2014
Building Pillars of MesoScale Particles on a Surface
Let’s build layers of (macromolecular) material on a surface. If the material were lego pieces, then the amount of coverage at any given point would be precisely the number that fell there and connected (interlocked). In the simplest cases, you might imagine the same type of construction on a macromolecular scale surface with nano sized bricks. The trouble is that at that small length scale, particles are no longer rigid. The possibility of such softness allows for higher density layers and even a lack of certainty as to which layer you are in. This leads to a roughness in the surface due to the fact that the soft legos stack more in some places than others. Moreover, there will be regions without any legos at all which means that the surface will not be completely covered. This led us to ask: What is the surface coverage as a function of the amount of macromolecular material coated on the surface and the degree of softness of that material?
As in our recent work using tricked-up hard particles, we wondered whether we could answer this question without using explicit soft particle interactions. It does, indeed, appear to work in the sense that we are able to capture the differences in coverage of the surface between a metastable coverage in which particles once trapped at a site remain there, and the relaxed coverage in which particles are allowed to spread across the surface. We also found that relaxation leads to reduced coverage fractions rather than larger coverage as one might have expected a spreading of particles due to the relaxation.
This work was performed by my graduate student, Dr. Galen Craven, in collaboration with a research scientist in my group, Dr. Alex Popov. The title of the article is "Effective surface coverage of coarse grained soft matter.” The work was funded by the NSF. It was published on-line in J. Phys. Chem. B back in July, and I’ve been waiting to write this post hoping that it would hit the presses. Unfrotunately, it’s part of a Special Issue on Spectroscopy of Nano- and Biomaterials which hasn’t quite yet been published. But I hope that it will be soon! Click on the doi link to access the article.
As in our recent work using tricked-up hard particles, we wondered whether we could answer this question without using explicit soft particle interactions. It does, indeed, appear to work in the sense that we are able to capture the differences in coverage of the surface between a metastable coverage in which particles once trapped at a site remain there, and the relaxed coverage in which particles are allowed to spread across the surface. We also found that relaxation leads to reduced coverage fractions rather than larger coverage as one might have expected a spreading of particles due to the relaxation.
This work was performed by my graduate student, Dr. Galen Craven, in collaboration with a research scientist in my group, Dr. Alex Popov. The title of the article is "Effective surface coverage of coarse grained soft matter.” The work was funded by the NSF. It was published on-line in J. Phys. Chem. B back in July, and I’ve been waiting to write this post hoping that it would hit the presses. Unfrotunately, it’s part of a Special Issue on Spectroscopy of Nano- and Biomaterials which hasn’t quite yet been published. But I hope that it will be soon! Click on the doi link to access the article.
Wednesday, September 10, 2014
OXIDE's Case for Inclusive Excellence in Chemistry
There's a catch-22 with any attempt to increase the participation of under-represented groups in the chemical sciences, and perhaps elsewhere too. On the one hand, students need to be trained to enter the workforce. On the other hand, they need to make the choice to be trained, presumably because jobs in the chemical sciences are more desirable than the alternatives. There exist many successful programs aimed at the former, but I believe that such programs face an uphill climb because, on top of everything else, they must convince potential candidates that a career path in the chemical sciences is desirable. Sadly, though the odds for success in professional sports are much lower, it appears to be relatively easy to convince someone to follow that dream through college. When it comes to choosing college majors, however, students tend to be more cautious about potential long-term employment opportunities. This is true for everyone, but it's often acutely so for students from under-represented groups who face the possibility of finding diversity inequities along their career path. An objective of the OXIDE effort is to help break the catch-22 by changing the culture so as to eliminate real or perceived diversity inequities. Our hypothesis is that a culture that is accommodating to everyone will lead to departments with demographics that are in congruence with those of the national population.
Our recent article, "A Top-Down Approach for Increasing Diversity and Inclusion in Chemistry Departments," was roughly based on an invited presentation (by the same title) at the Presidential Symposium held at the 246th National Meeting of the American Chemical Society (ACS) in Indianapolis in September 2013. The symposium topic was the "Impact of Diversity and Inclusion" and included several other presentations that were also turned into peer reviewed articles in the recently published ACS Symposium book. I spoke about the work by Shannon Watt and me through our OXIDE program. She's pictured just to my left in the image accompanying this post. That picture, by the way, was actually taken at the 248th ACS National Meeting held in San Francisco in August 2014. I included this more recent photo with this post because, in addition to Shannon and me, it features several individuals who have been supportive of the OXIDE effort and who also spoke at the recent symposium on "Advancing the Chemical Sciences through Diversity" and thereby continued the dialogue on inclusive excellence at ACS meetings.
The article was written with my collaborator, Shannon Watt. The reference is "A top-down approach for diversity and inclusion in chemistry departments," Careers, Entrepreneurship, and Diversity: Challenges and Opportunities in the Global Chemistry Enterprise, ACS Symposium Series 1169, edited by H. N. Cheng, S. Shah, and M. L. Wu, Chapter. 19, pp. 207-224 (American Chemical Society, Washington DC, 2014). (ISBN13: 9780841229709, eISBN: 9780841229716, doi: 10.1021/bk-2014-1169.ch019) Our OXIDE work is funded by the National Science Foundation, the Department of Energy and the National Institutes of Health through NSF grant #CHE-1048939.
We are also grateful to recent gifts from the Dreyfus Foundation, the Research Corporation for Science Advancement, and a private donor.
Our recent article, "A Top-Down Approach for Increasing Diversity and Inclusion in Chemistry Departments," was roughly based on an invited presentation (by the same title) at the Presidential Symposium held at the 246th National Meeting of the American Chemical Society (ACS) in Indianapolis in September 2013. The symposium topic was the "Impact of Diversity and Inclusion" and included several other presentations that were also turned into peer reviewed articles in the recently published ACS Symposium book. I spoke about the work by Shannon Watt and me through our OXIDE program. She's pictured just to my left in the image accompanying this post. That picture, by the way, was actually taken at the 248th ACS National Meeting held in San Francisco in August 2014. I included this more recent photo with this post because, in addition to Shannon and me, it features several individuals who have been supportive of the OXIDE effort and who also spoke at the recent symposium on "Advancing the Chemical Sciences through Diversity" and thereby continued the dialogue on inclusive excellence at ACS meetings.
The article was written with my collaborator, Shannon Watt. The reference is "A top-down approach for diversity and inclusion in chemistry departments," Careers, Entrepreneurship, and Diversity: Challenges and Opportunities in the Global Chemistry Enterprise, ACS Symposium Series 1169, edited by H. N. Cheng, S. Shah, and M. L. Wu, Chapter. 19, pp. 207-224 (American Chemical Society, Washington DC, 2014). (ISBN13: 9780841229709, eISBN: 9780841229716, doi: 10.1021/bk-2014-1169.ch019) Our OXIDE work is funded by the National Science Foundation, the Department of Energy and the National Institutes of Health through NSF grant #CHE-1048939.
We are also grateful to recent gifts from the Dreyfus Foundation, the Research Corporation for Science Advancement, and a private donor.
Friday, August 22, 2014
Pitching molecular fastballs through a hurricane
The first course I taught at Georgia Tech was a graduate course in statistical mechanics. I quickly discovered that a big barrier for my chemistry students was a lack of understanding of modern classical mechanics. Actually, this has also been a problem for them in learning quantum mechanics too. It might seem odd because classical mechanics is everywhere around you. It is simply the theory that describes the motion of baseballs, pool balls, and rockets. The details of the theory get amazingly complicated as you try to address interactions between the particles. Baseballs don't have such long range interactions so no worries there. But molecules do, and these interactions need to be included to understand the full complexity of molecules in classical (and quantum) mechanical regimes. In order to address this gap, I created a primer on classical mechanics that I have been sharing with my students ever since. But this document was not available to anyone beyond my classroom. So when I was asked to submit a review article on modern molecular dynamics, it was a no-brainier to include my primer at the front of the article so as to bring up readers up to speed on the classical equations of motion beyond Newton's day.
We didn't stop there, of course. The advanced review article, written with my collaborator, Dr. Alex Popov, also describes modern theoretical and computational methods for describing the motion of molecules in extreme environments far from equilibrium (like, for example, a hurricane). There are lots of ways in which this problem involves many moving parts (that is, many variables or degrees of freedom.). One of these involves the approximate separation in the characteristic scales of electrons and nuclei for which the 2013 Nobel Prize in Chemistry was, in part, awarded. Another involves the three-dimensional variables required to describe each atom in every molecule for which there usually are many. Think moles not scores. This increasing complexity gives rise to feedback loops that can explode (just like bad feedback on a microphone-speaker set-up), collapse or do something in between depending on very subtle balances in the interactions. Slowly, but surely, the scientific community is beginning to understand how to address these complex multiscale systems far from equilibrium. In our recent article, we reviewed the current state of the art in this area in the hope that it can help us and others advance the field.
The article was written in collaboration with Alexander V. Popov. The title is "Molecular dynamics out of equilibrium: Mechanics and measurables" and our work was funded by the National Science Foundation. It was recently made available on-line in WIREs Comput. Mol. Sci. 10.1002/wcms.1190, "Early View" (2014), and should be published soon.
We didn't stop there, of course. The advanced review article, written with my collaborator, Dr. Alex Popov, also describes modern theoretical and computational methods for describing the motion of molecules in extreme environments far from equilibrium (like, for example, a hurricane). There are lots of ways in which this problem involves many moving parts (that is, many variables or degrees of freedom.). One of these involves the approximate separation in the characteristic scales of electrons and nuclei for which the 2013 Nobel Prize in Chemistry was, in part, awarded. Another involves the three-dimensional variables required to describe each atom in every molecule for which there usually are many. Think moles not scores. This increasing complexity gives rise to feedback loops that can explode (just like bad feedback on a microphone-speaker set-up), collapse or do something in between depending on very subtle balances in the interactions. Slowly, but surely, the scientific community is beginning to understand how to address these complex multiscale systems far from equilibrium. In our recent article, we reviewed the current state of the art in this area in the hope that it can help us and others advance the field.
The article was written in collaboration with Alexander V. Popov. The title is "Molecular dynamics out of equilibrium: Mechanics and measurables" and our work was funded by the National Science Foundation. It was recently made available on-line in WIREs Comput. Mol. Sci. 10.1002/wcms.1190, "Early View" (2014), and should be published soon.
Wednesday, August 20, 2014
LiCN taking a dip in an Ar bath
We all know that it’s easier to move through air than water. Changing the environment to molasses means that you’ll move even slower. Thus it’s natural to think that the thicker (denser) the solvent (bath), the slower a particle will swim through it. More precisely, what matters is not the density but the degree to which the moving particle interacts with the solvent, and this can be described through the friction between the particle and the fluid. Chemical reactions have long known to be increasingly slower with increasing friction. The problem with this seemingly simple concept is that Kramers showed long ago that there exists a regime (when the surrounding fluid is very weakly interacting with the particle) in which the reactions actually speed up with increasing friction. This crazy regime arises because reactants need energy to surmount the barriers leading to products, and they are unable to get this energy from the solvent if their interaction is very weak. A small increase of this weak interaction facilitates the energy transfer, and voila the reaction rate increases. What Kramers didn’t find is a chemical reaction which actually exhibits this behavior, and the hunt for such a reaction has long been on…
A few years ago, my collaborators in Madrid and I found a reaction that seems to exhibit a rise and fall in chemical rates with increasing friction. (I wrote about one of my visits to my collaborators in Madrid in a previous post.) It involves the isomerization reaction from LiCN to CNLi where the lithium is initially bonded to the carbon, crosses a barrier and finally bonds to the nitrogen on the other side. We placed it inside an argon bath and used molecular dynamics to observe the rate. Our initial work fixed the CN bond length because that made the simulation much faster and we figured that the CN vibrational motion wouldn’t matter much. But the nagging concern that the CN motion might affect the results remained. So we went ahead and redid the calculations releasing the constraint on the CN motion. I’m happy to report that the rise and fall persisted. As such the LiCN isomerization reaction rate is fastest when the density of the Argon bath is neither too small nor too large, but rather when it is just right.
The article with my collaborators, Pablo Garcia Muller, Rosa Benito and Florentino Borondo was just published in the Journal of Chemical Physics 141, 074312 (2014), and may be found at this doi hyperlink. This work was funded by the NSF on the American side of the collaboration, by Ministry of Economy and Competiveness-Spain and ICMAT Severo Ochoa on the Spanish side, and by the EU’s Seventh Framework People Exchange programme.
A few years ago, my collaborators in Madrid and I found a reaction that seems to exhibit a rise and fall in chemical rates with increasing friction. (I wrote about one of my visits to my collaborators in Madrid in a previous post.) It involves the isomerization reaction from LiCN to CNLi where the lithium is initially bonded to the carbon, crosses a barrier and finally bonds to the nitrogen on the other side. We placed it inside an argon bath and used molecular dynamics to observe the rate. Our initial work fixed the CN bond length because that made the simulation much faster and we figured that the CN vibrational motion wouldn’t matter much. But the nagging concern that the CN motion might affect the results remained. So we went ahead and redid the calculations releasing the constraint on the CN motion. I’m happy to report that the rise and fall persisted. As such the LiCN isomerization reaction rate is fastest when the density of the Argon bath is neither too small nor too large, but rather when it is just right.
The article with my collaborators, Pablo Garcia Muller, Rosa Benito and Florentino Borondo was just published in the Journal of Chemical Physics 141, 074312 (2014), and may be found at this doi hyperlink. This work was funded by the NSF on the American side of the collaboration, by Ministry of Economy and Competiveness-Spain and ICMAT Severo Ochoa on the Spanish side, and by the EU’s Seventh Framework People Exchange programme.
Monday, August 18, 2014
Taming the multiplicity of pathways in the restructuring of proteins
The average work to move molecular scale objects closer together or further apart is difficult to predict because the calculation depends on the many other objects in the surroundings. For example, you might find if easy to cross a four-lane street of grid-locked cars (requiring a small amount of work) but nearly impossible to cross a highway with cars speeding by at 65mph (requiring a lot of work.) When the system is a protein whose overall structure is expanded or contracted, there exist a myriad possible configurations which must be included to obtain the average required work. Such a calculation is computationally expensive and likely inefficient. Instead, we have been using a method (steered molecule dynamics) developed by Schulten and coworkers based on Jarzynski's equality. It helps us compute the equilibrium work using (driven) paths far outside of equilibrium. The trouble is that the surroundings get in the way and drive the system along paths that get out of bounds quickly.
In previous work, we tamed these naughty paths by reigning them all in to a tighter region of structures. Unfortunately, this may be too aggressive. The key is to realize that not all paths are naughty. That is, that there may be more than one region of structures that contribute significantly to the calculation of the work. We found that we could include such not-quite-naughty paths and still maintain the efficiency of our adaptive steered molecular dynamics. I described the early work on ASMD in my recent ACS Webinar.
This research project was truly performed in collaboration. My recent graduate student, Gungor Ozer, did the work while he was a postdoc at Boston University. Tom Keyes hosted him there and gave great insight on the sampling approach. Stephen Quirk, as always, grounded us in the biochemistry.
The title of the article is "Multiple branched adaptive steered molecular dynamics.” The work was funded by the NSF. It was just released at J. Chem. Phys. 141, 064101 (2014). Click on the JCP link to access the article.
In previous work, we tamed these naughty paths by reigning them all in to a tighter region of structures. Unfortunately, this may be too aggressive. The key is to realize that not all paths are naughty. That is, that there may be more than one region of structures that contribute significantly to the calculation of the work. We found that we could include such not-quite-naughty paths and still maintain the efficiency of our adaptive steered molecular dynamics. I described the early work on ASMD in my recent ACS Webinar.
This research project was truly performed in collaboration. My recent graduate student, Gungor Ozer, did the work while he was a postdoc at Boston University. Tom Keyes hosted him there and gave great insight on the sampling approach. Stephen Quirk, as always, grounded us in the biochemistry.
The title of the article is "Multiple branched adaptive steered molecular dynamics.” The work was funded by the NSF. It was just released at J. Chem. Phys. 141, 064101 (2014). Click on the JCP link to access the article.
Saturday, August 9, 2014
Lost in Projection
Life is full of cycles. For the American Chemical Society (ACS), there’s a National Meeting held every 6 months. With that as my time constant, it feels as if it was just the other day that I gave the Keynote Lecture at the ACS Committee on Minority Affairs Luncheon at the last National Meeting in Dallas. Last March, I framed my remarks through the title of this post. I knew that it was a good title because my wife liked it. But she also wondered what in the world I would talk about under such a title. This seemed fortuitous. It meant that I could say nearly anything without disappointing the audience. After all, they couldn't possibly have any expectations. In truth, the title wasn’t advertised as evidence by the large turnout.
What is lost in the projection of how others frame you? That is, there is a potential disconnect between what others expect of you and the person who you believe yourself. To what extent are you bound by what others expect of you? Such expectations may arise from how others have seen you in the past or perhaps by who they see on first impression. I came to the United States as a child, a Cuban immigrant who spoke no English but proud to be able to count from one to ten without too much of an accent. Children grow and learn so surely I cannot be bound by that early frame. As adults, we may not grow physically, but we also learn. And yet, we have a tendency to hold others to the frames that we first see of them. Even more dangerous to each of us is the fact that the frame is often projected onto us by others, and not always correctly. There were several young scientists in the room. During the question and answer period, they echoed the angst of having felt bound by such projections in the past. I hope that I succeeded in encouraging them to find their way in breaking those projections and choosing their own frame.
The cycle continues this weekend with the start of the ACS meeting in San Francisco. If you are here, I encourage you to go to Monday’s CMA Luncheon where Madeleine Jacobs will be speaking. The issue of implicit bias and the effects that has on academic careers will also be a large piece of the Symposium on Advancing the Chemical Sciences Through Diversity to be held at the Hilton San Francisco Union Square Hotel all-day on Tuesday. I hope to see you there!
What is lost in the projection of how others frame you? That is, there is a potential disconnect between what others expect of you and the person who you believe yourself. To what extent are you bound by what others expect of you? Such expectations may arise from how others have seen you in the past or perhaps by who they see on first impression. I came to the United States as a child, a Cuban immigrant who spoke no English but proud to be able to count from one to ten without too much of an accent. Children grow and learn so surely I cannot be bound by that early frame. As adults, we may not grow physically, but we also learn. And yet, we have a tendency to hold others to the frames that we first see of them. Even more dangerous to each of us is the fact that the frame is often projected onto us by others, and not always correctly. There were several young scientists in the room. During the question and answer period, they echoed the angst of having felt bound by such projections in the past. I hope that I succeeded in encouraging them to find their way in breaking those projections and choosing their own frame.
The cycle continues this weekend with the start of the ACS meeting in San Francisco. If you are here, I encourage you to go to Monday’s CMA Luncheon where Madeleine Jacobs will be speaking. The issue of implicit bias and the effects that has on academic careers will also be a large piece of the Symposium on Advancing the Chemical Sciences Through Diversity to be held at the Hilton San Francisco Union Square Hotel all-day on Tuesday. I hope to see you there!
Wednesday, July 30, 2014
Getting to the shore when riding a wave from reactant to product
Suppose that you were trying to get across a floor that goes side to side like a wave goes up and down. If you started on the start line (call it the reactants), how long would it take you to cross to the finish line (call it the products) on the other side? In earlier work, we found “fixed” structures that could somehow tell you exactly when you were on the reactant and product sides even while the barrier was waving side to side. Just like you and I would avoid getting seasick while riding such a wave, the “fixed” structure has to move, but just not as much as the wave. So the structure of our dividing surface is “fixed” in the sense that our planet is always on the same orbit flying around the sun. This latter analogy can’t be taken too far because we know that our planet is thankfully stable. In the molecular case, the orbit is not stable. We just discovered that the rates at which molecular reactants move away from the dividing surface can be related to the reaction rate between reactant and products in a chemical reaction. (Note that the former rates are called Floquet exponents.) This is a particularly cool advance because we are now able to relate the properties of the moving dividing surface directly to chemical reactions, at least for this one simplified class of reactions.
The work involved a collaboration with Galen Craven from my research group and Thomas Bartsch from Loughborough University. The title of the article is "Communication: Transition state trajectory stability determines barrier crossing rates in chemical reactions induced by time-dependent oscillating fields.” The work was funded by the NSF, and the international partnership (Trans-MI) was funded by the EU People Programme (Marie Curie Actions). It was just released as a Communication at J. Chem. Phys. 141, 041106 (2014). Click on the JCP link to access the article.
The work involved a collaboration with Galen Craven from my research group and Thomas Bartsch from Loughborough University. The title of the article is "Communication: Transition state trajectory stability determines barrier crossing rates in chemical reactions induced by time-dependent oscillating fields.” The work was funded by the NSF, and the international partnership (Trans-MI) was funded by the EU People Programme (Marie Curie Actions). It was just released as a Communication at J. Chem. Phys. 141, 041106 (2014). Click on the JCP link to access the article.
Friday, July 25, 2014
When the buffalo roam, do they go over the pass or across the plain?
In one of our papers from last year (recapped in an earlier post), we found that in at least one chemical reaction (the ketene isomerization), the reaction could involve rather distinct pathways. On the one hand, the system could go across the break between the two energetic mountains separating the reactant and product. On the other hand, it could find a flat plain in which it could meander slowly across. The first of these two cases involves a narrow pass that is difficult for it to get through. The other is a wide plain but it costs a lot of energy to get up to it. Chemical reaction rate theory is built on the notion that the reaction always goes across the narrow passage as long as it’s the easiest one to get over. However, in the last decade there has been a lot of work observing that roaming over the flat plain has its privileges.
My postdoctoral student, Inga Ulusoy, and I wondered whether the ketene reaction gave rise to both possible classes of paths. It did! We also wondered the degree to which each path affected the rate in which the molecule reacted. We found earlier that the traditional pathway (over the break between the barriers) was the most important one in a model of the reaction with only two degrees of freedom. This led us and others to question whether our result was an accident of the simplicity of our model. In our recent paper, we extended the model to a larger number of degrees of freedom. Interestingly, the main result was the same. Namely, the reacting partners still have the possibility of roaming, but the direct path over the break between the barriers is still the most important one.
The article, "Revisiting roaming trajectories in ketene isomerization at higher dimensionality,” was recently published at Theoretical Chemistry Accounts 133, 1528 (2014). (doi:10.1007/s00214-014-1528-z) The work was supported by the AFOSR. Equally, importantly, it was a real treat to include our work in an issue published in honor of Greg Ezra's 60th birthday. I have followed his work since I was a graduate student, and have learned much from it. While science is immutable, it’s the fact that people are involved in the discovery that makes it humane. And for this reason, it’s particularly fun to be able to contribute to issues that honor the people involved in advancing science.
My postdoctoral student, Inga Ulusoy, and I wondered whether the ketene reaction gave rise to both possible classes of paths. It did! We also wondered the degree to which each path affected the rate in which the molecule reacted. We found earlier that the traditional pathway (over the break between the barriers) was the most important one in a model of the reaction with only two degrees of freedom. This led us and others to question whether our result was an accident of the simplicity of our model. In our recent paper, we extended the model to a larger number of degrees of freedom. Interestingly, the main result was the same. Namely, the reacting partners still have the possibility of roaming, but the direct path over the break between the barriers is still the most important one.
The article, "Revisiting roaming trajectories in ketene isomerization at higher dimensionality,” was recently published at Theoretical Chemistry Accounts 133, 1528 (2014). (doi:10.1007/s00214-014-1528-z) The work was supported by the AFOSR. Equally, importantly, it was a real treat to include our work in an issue published in honor of Greg Ezra's 60th birthday. I have followed his work since I was a graduate student, and have learned much from it. While science is immutable, it’s the fact that people are involved in the discovery that makes it humane. And for this reason, it’s particularly fun to be able to contribute to issues that honor the people involved in advancing science.
Monday, July 14, 2014
Inclusive excellence symposium coming up at ACS San Francisco
Two qualifiers, successful and diverse, for the research enterprise are inseparable concepts. And yet, some members in the scientific community have often treated them as orthogonal if not outright destructively interfering. (Please forgive my geek speak here!) I think that the only part that is destructive is the failure to be inclusive. Indeed, as we are broadening participation in the chemical research enterprise, we are drawing more and better talent from all over the world and this should include that which is within our borders. Unfortunately, we aren't quite there yet as is visible through the existing imbalance in the demographics between the US population and the chemical workforce. Achieving parity requires us to be actively engaged.
To this end, I just published a Comment in C&EN to advertise the upcoming symposium on "Advancing the Chemical Sciences Through Diversity in Participation." (Earlier, I wrote on this blog about why I publish there and here. You can also check out the ChemDiversity Blog post advertising the symposium.) If you happen to be in San Francisco in mid August, feel free to join us at the Hilton near Union Square. You'll learn a few tips for advancing inclusive excellence and you'll be in the middle of San Francisco. Hard to beat that pairing!
Check out the Comment on page 45 of the July 14, 2014 C&EN at this link. (Apologies if it's closed to ACS members and subscribers only.)
To this end, I just published a Comment in C&EN to advertise the upcoming symposium on "Advancing the Chemical Sciences Through Diversity in Participation." (Earlier, I wrote on this blog about why I publish there and here. You can also check out the ChemDiversity Blog post advertising the symposium.) If you happen to be in San Francisco in mid August, feel free to join us at the Hilton near Union Square. You'll learn a few tips for advancing inclusive excellence and you'll be in the middle of San Francisco. Hard to beat that pairing!
Check out the Comment on page 45 of the July 14, 2014 C&EN at this link. (Apologies if it's closed to ACS members and subscribers only.)
Saturday, July 12, 2014
On my experience delivering a webinar...
I recently participated as a speaker in a Webinar for the American Chemical Society (ACS.) It was only the second webinar that I have delivered. My first was held on January 2013 as part of the monthly meeting series of the Lehigh Valley Local section of the ACS. They were an early adopter of the medium. That is, they were quick to figure out that it's cost effective to host speakers from a distance while also addressing a greater number of their members. The latter is particularly important to them because they cover a large geographic area placing any particular choice of meeting location too far from most of their members. My host, Lorena Tribe, helped me learn how to use questions through the presentation effectively in order to engage their web audience. I found the technique to be so successful that I have retained and used the questions (in think-pair-share style) as I present our work (on the energetics of proteins) at department seminars.
As a consequence, when I was asked to participate in the ACS Webinar, I was initially not phased by the opportunity. That is, until I learned that the audience would include nearly 400 participants. Fortunately, the ACS staff was similarly awesome. They provided all the necessary infrastructure and great user support. All I had to do was put my slides together just like I do for any other seminar. The inclusion of my industrial collaborator, Stephen Quirk, framed my otherwise academic discussion into one that was more accessible for a broader (viz. industrial) audience. Plus he did all the hard work of selecting the questions for me to answer during the Q and A. All-in-all my total time investment was probably less than four hours. Moreover, we reached a large audience and one that I probably would not have "seen" otherwise. That's a high benefit to cost ratio which I consider a big win.
If you missed my Webinar on "Digitally Pulling Proteins: Molecular Dynamics Simulations," you might still be able to hear it at http://acswebinars.org/digital-proteins. At present, it's available only for view by ACS members.
As a consequence, when I was asked to participate in the ACS Webinar, I was initially not phased by the opportunity. That is, until I learned that the audience would include nearly 400 participants. Fortunately, the ACS staff was similarly awesome. They provided all the necessary infrastructure and great user support. All I had to do was put my slides together just like I do for any other seminar. The inclusion of my industrial collaborator, Stephen Quirk, framed my otherwise academic discussion into one that was more accessible for a broader (viz. industrial) audience. Plus he did all the hard work of selecting the questions for me to answer during the Q and A. All-in-all my total time investment was probably less than four hours. Moreover, we reached a large audience and one that I probably would not have "seen" otherwise. That's a high benefit to cost ratio which I consider a big win.
If you missed my Webinar on "Digitally Pulling Proteins: Molecular Dynamics Simulations," you might still be able to hear it at http://acswebinars.org/digital-proteins. At present, it's available only for view by ACS members.
Wednesday, June 18, 2014
Soft materials made up of tricked-up hard particles
Materials are made of smaller objects which in turn are made of smaller objects which in turn… For chemists, this hierarchy of scales usually stops when you eventually get down to atoms. However, well before that small scale, we treat some of these objects as particles (perhaps nano particles or colloids) that are clearly distinguishable and whose interactions may somehow be averaged (that is, coarse-grained) over the smaller scales. This gives rise to all sorts of interesting questions about how they are made and what they do once made. One of these questions concerns the structure and behavior of these particles if their mutual interactions is soft, that is when they behave as squishy balls when they get close to each other and unlike squishy balls continue to interact even when they are far away. This is quite different from hard interactions, that is when they behave like billiard balls that don’t overlap but don’t feel each other when they aren’t touching.
I previously blogged about our work showing that in one-dimension, we could mimic the structure of assemblies of soft particles using hard particles if only the latter were allowed to overlap (ghostlike) with some prescribed probability. In one dimension, this was like looking at a system of rods on a line. We wondered whether this was also possible in two dimensions (disks floating on a surface) or in three dimensions (balls in space). In our recent article, we confirmed that this overlapping (i.e. interpenetrable) hard-sphere model does indeed mimic soft particles in all three dimensions. This is particularly nice because the stochastic hard-sphere model is a lot easier to simulate and to solve using theoretical/analytical approaches. For example, we found a formula for the effective occupied volume directly from knowing the “softness” in the stochastic hard-sphere model.
The work was done in collaboration with my group members, Galen Craven and Alexander V. Popov. The title is "Structure of a tractable stochastic mimic of soft particles" and the work was funded by the National Science Foundation. It was released just this week at Soft Matter, 2014, Advance Article (doi:10.1039/C4SM00751D). It's already available as an Advance Article on the RSC web site, though this link should remain valid once it is formally printed.
I previously blogged about our work showing that in one-dimension, we could mimic the structure of assemblies of soft particles using hard particles if only the latter were allowed to overlap (ghostlike) with some prescribed probability. In one dimension, this was like looking at a system of rods on a line. We wondered whether this was also possible in two dimensions (disks floating on a surface) or in three dimensions (balls in space). In our recent article, we confirmed that this overlapping (i.e. interpenetrable) hard-sphere model does indeed mimic soft particles in all three dimensions. This is particularly nice because the stochastic hard-sphere model is a lot easier to simulate and to solve using theoretical/analytical approaches. For example, we found a formula for the effective occupied volume directly from knowing the “softness” in the stochastic hard-sphere model.
The work was done in collaboration with my group members, Galen Craven and Alexander V. Popov. The title is "Structure of a tractable stochastic mimic of soft particles" and the work was funded by the National Science Foundation. It was released just this week at Soft Matter, 2014, Advance Article (doi:10.1039/C4SM00751D). It's already available as an Advance Article on the RSC web site, though this link should remain valid once it is formally printed.
Saturday, June 7, 2014
Old-World Publishing in the New Age
Through the web, we can self-publish pretty much anything at any time. This doesn't guarantee, however, that anyone will read it. Actually no venue can guarantee that. Old world platforms such as newspapers, trade publications and journals do have a circulation among their audiences that effectively guarantee a certain number of page views. On the other hand, all-electronic open access journals can serve as such amplifiers as well. Some blogs have become so popular that their number of page views exhibit viral-like growth. So why should anyone publish on the old-world platforms?
That's a loaded question, and truly one that has many possible good arguments to support it. I'll rest on providing one answer by example. I recently published a Comment in C&EN. The piece was quite a bit longer than my usual blog post. As such, it would have been appropriate for my EveryWhereChem blog only if I broke it up into about three posts. There is one more key difference. I was able to work with an editor who helped me to focus the piece while allowing me to retain my "voice." My prose was probably a bit too breezy, but she embraced it and made it better. Trouble is that editors need to be paid and one might argue that authors do too! While this and other quality control mechanisms are not exclusive features of the old-world publishing model, they are certainly a large part of the service that authors and readers enjoy from them. They also serve as curators of the pieces that they publish. And this means that a good editor can exert a meta-level quality control that adds value to the readership. There's also a role for blogging as otherwise I wouldn't be writing this too. My postmodern view of the so-called traditional publishing venues is that they remain valuable even if we aren't sure how to monetize it as readily as we once did.
My C&EN Comment focused on Mentoring and the key role if fills in advancing young scientists into their careers. Most new faculty learn the job on the job. As the demands and the tenure decision pressure have grown, it is nearly impossible to figure out the job without help. This is where mentoring can play a big role. The New Faculty Workshop is one attempt to institutionalize mentoring across all of the chemistry research- active departments. I wrote about my experience at last year's New Faculty Workshop in two earlier posts on July 6 and July 16. The next workshop being held on July 31-August 2, and I'm looking forward to meeting the newest cohort of young faculty.
Check out my my March 24th Comment in C&EN on “Mentoring New Faculty—It Really Works!” and John Schwab’s letter to the editor on May 19th reiterating the “Importance of Mentoring” in response to my Comment.
That's a loaded question, and truly one that has many possible good arguments to support it. I'll rest on providing one answer by example. I recently published a Comment in C&EN. The piece was quite a bit longer than my usual blog post. As such, it would have been appropriate for my EveryWhereChem blog only if I broke it up into about three posts. There is one more key difference. I was able to work with an editor who helped me to focus the piece while allowing me to retain my "voice." My prose was probably a bit too breezy, but she embraced it and made it better. Trouble is that editors need to be paid and one might argue that authors do too! While this and other quality control mechanisms are not exclusive features of the old-world publishing model, they are certainly a large part of the service that authors and readers enjoy from them. They also serve as curators of the pieces that they publish. And this means that a good editor can exert a meta-level quality control that adds value to the readership. There's also a role for blogging as otherwise I wouldn't be writing this too. My postmodern view of the so-called traditional publishing venues is that they remain valuable even if we aren't sure how to monetize it as readily as we once did.
My C&EN Comment focused on Mentoring and the key role if fills in advancing young scientists into their careers. Most new faculty learn the job on the job. As the demands and the tenure decision pressure have grown, it is nearly impossible to figure out the job without help. This is where mentoring can play a big role. The New Faculty Workshop is one attempt to institutionalize mentoring across all of the chemistry research- active departments. I wrote about my experience at last year's New Faculty Workshop in two earlier posts on July 6 and July 16. The next workshop being held on July 31-August 2, and I'm looking forward to meeting the newest cohort of young faculty.
Check out my my March 24th Comment in C&EN on “Mentoring New Faculty—It Really Works!” and John Schwab’s letter to the editor on May 19th reiterating the “Importance of Mentoring” in response to my Comment.
Subscribe to:
Posts (Atom)